Reminder: as an Amazon Associate I earn small commissions from qualifying purchases made through some of the links below.
If you have been reading about coffee extraction lately, you might be familiar with the term astringencywhich is often used to describe poorly extracted coffee. This is a descriptive for which the meaning is generally not well known, and it’s worth pondering why we dislike it so much in the specialty coffee world, contrary to other communities like wine and beer.
First, let me try to describe what astringency feels like. Astringency creates a dryness sensation in your mouth, and generally mutes a lot of other flavors, especially when it’s strong. It can often be found in fruits or vegetables, especially unripe ones − in fact, the astringency that is present in wine comes from the grape’s seeds and skin (Mattivi et al. 2009). If you ever ate an unripe banana and could not resist from grinning as the dryness sensation invaded your whole face, then you might know what I’m talking about. The one thing I recall being the most astringent I have experienced was the spongy white pulp separating seeds inside of a pomegranate. Another good example of astringency is over-extracted green tea, but it also contains other strong flavors so it’s not the most precise example.
Astringency is not seen as a necessarily bad thing by wine experts, and I suspect that this is due at least in part to red wine being much more concentrated than filter coffee; typically wine has total dissolved solids concentrations between 1.7% and 3.0% compared to 1.3% to 1.5% for filter coffee (e.g. see Schopfer & Lipka, 1973 and this French post), so astringency won’t easily mask everything else, and can instead be in balance with the global taste profile. In tea and coffee, we try to get rid of astringency as much as we can because it can easily become the dominant sensation, and can even come close to being the only perceptible one.
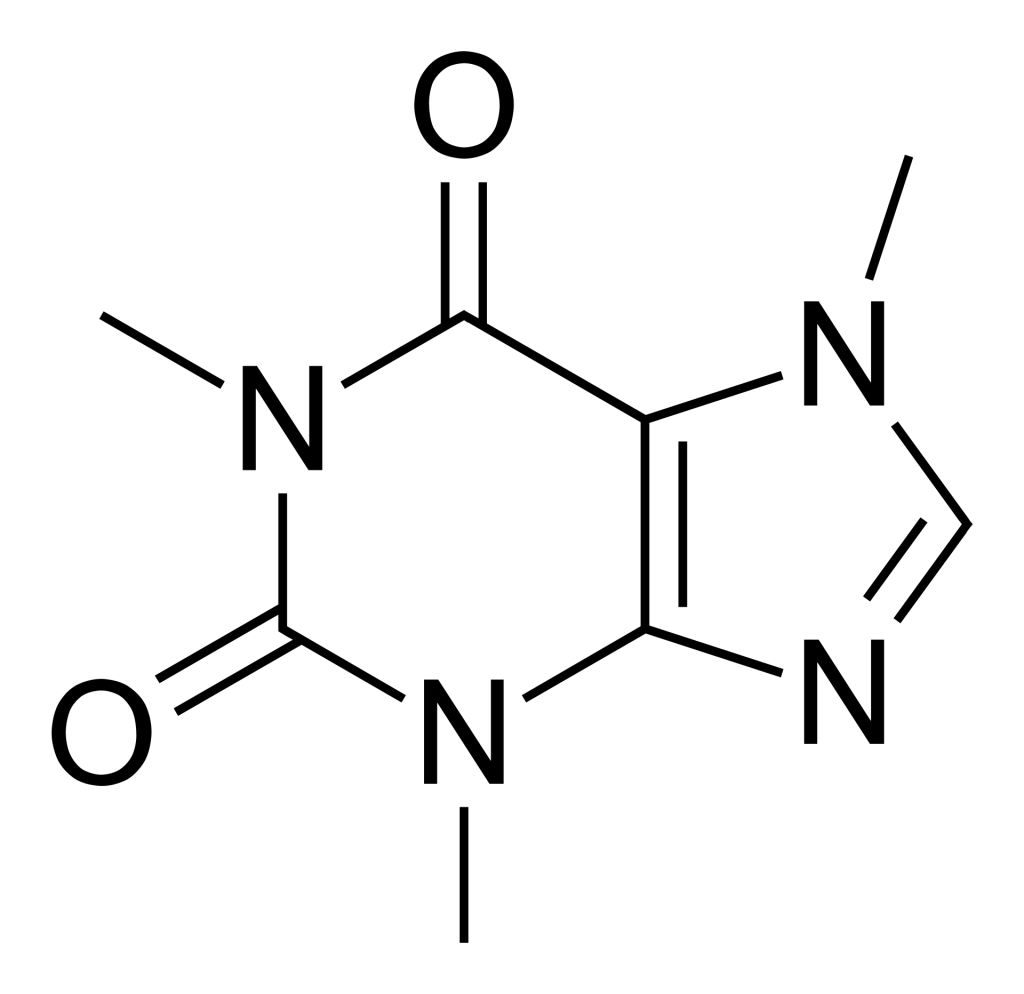
The astringency sensation is caused by soluble organic compounds that belong to a class called polyphenols, which includes tannins but also other complex molecules. They are often produced by plants as a defense mechanism against insects and other predators. These complex and large molecules can attach to proteins like Lego blocks, and form large clusters that can precipitate out of solution. This is what happens when we feel astringency; the polyphenols bind to proteins in our saliva and precipitate, forming clumps that inhibit our taste buds’ ability to taste the coffee properly, and cause this rugged and dry sensation.
Fortunately for us, polyphenols are mostly very large molecules, which makes them heavier and harder to extract from coffee particles, compared to smaller molecules like caffeine and the various acids we enjoy. This makes it possible to extract the good molecules, while avoiding the polyphenols that cause this astringent sensation. If you prepare coffee by immersion, you likely won’t often encounter astringency, because the immersion method extracts coffee solubles more gently, especially if you grind coarse and don’t agitate too much. Furthermore, the extraction happens in a relatively uniform way, in other words you won’t have large amounts of fresh water extracting a small amount of coffee particles. Remember that fresh water is a much more potent solvent compared to concentrated water. However, immersion brews also never achieve a very good filtration of coffee fines and other insoluble compounds.


If you happen to prefer the taste of coffee without these insoluble particles as I do, you might prefer coffee prepared by the percolation method, where fresh water is poured on a bed of coffee, filtering out any undissolved solids (see this previous post for a more detailed discussion of the differences between percolation and immersion). Percolation brews are however much more prone to causing astringency, because channels can form where a larger fraction of water passes through preferential paths, which can over-extract some small regions of the coffee bed (we sometimes call this local over-extraction). This allows heavier polyphenols to be extracted, and makes the resulting brew astringent.
One thing I do not know is whether polyphenols can be filtered out by the coffee bed itself. When preparing percolation brews, the coffee bed filters out a lot of undissolved solids, and prevents them from getting in your brew. This is why a coffee bed full of fines will only clog the paper filter if you agitate it a lot; if you don’t agitate it, then the coffee bed acts as a filter and retains these fines, preventing your paper filter from clogging. This is also why a V60 has much less undissolved solids than a typical Aeropress brew; the depth of the coffee bed in the latter is typically much smaller, so it lets more fines in your cup.
Based on experience, I know that a typical V60 coffee bed can filter out a lot more compounds than just a paper filter, even in the extreme case of Whatman Grade 5 paper filters that have average pore sizes of 2.5 micron; I’ll talk more about this in a future post. However, I’m not sure if coffee beds are such good filters that they would remove polyphenols that were extracted and dissolved in the slurry. I think it is unlikely, because although polyphenols can be much larger than other coffee compounds, they are still much smaller than a micron. For example, some wine tannins have sizes in the range 50−70 Å (McRae et al., 2014). If you are not familiar with these units, 1 micron is equivalent to 10,000 Angstrom (Å), so a coffee bed would need to be a much better filter filter than a Whatman Grade 5 paper filter to remove polyphenols.
Ifthe coffee bed is able to filter out polyphenols however, the presence of a large channel could provide an additional reason why they let polyphenols in our beverage, because they would create localized regions of bad filtration, where polyphenols and undissolved solids pass through. That is an interesting question to me, because it would mean that over-extraction could potentially be fixed by a good non-channeling coffee bed, even if polyphenols are getting extracted in the slurry for other reasons.
This whole idea of channels causing localized poor filtration made me want to directly measure the amount of fines and other undissolved solids in my coffee brews in an objective way. This can be done with the help of turbidity meters, but until about a month ago, I though those were extremely expensive equipment only built for labs. When I saw Ray Murakawa using what seemed like a portable turbidity meter on Instagram, I got very excited and he told me they are actually portable and affordable ! Thanks to the help of my Patreon supporters, I promptly ordered one and started measuring some of my brews. Turbidity meters work through a different principle than refractometers, but they give us information that is a bit similar. Refractometers inform us about the concentration of dissolved solids, and turbidity meters inform us about the concentration of undissolved solids.

When taking turbidity measurement, I realized something really interesting; the cloudiness goes up pretty fast as a brew cools down and stales. This was not too surprising at first because cooler water is less good at dissolving things, so we can expect the total amount of undissolved solids to increase as the brew cools. What I found really surprising is that even if you quickly cool down filter coffee to room temperature, its cloudiness keeps going up quite quickly for about half an hour, and then very slowly for more than 12 hours (I haven’t tried measuring older coffee). I’m not sure about this, but I suspect this increasing cloudiness at room temperature might be caused by polyphenols binding to some proteins that were also extracted from the coffee. Undoubtedly there are many other things that affect the absolute cloudiness of a coffee beverage, especially in an immersion, but it’s possible that the rate of increase at room temperature correlates with astringency. Furthermore, given that most of this turbidity increase happens within half an hour or so, I now wonder whether it’s related at all with coffee tasting bad after a brew stales.
Reading about polyphenols also made me realize there may be a reason beyond channeling why filter brew methods with very finely ground coffee (i.e., finer than espresso) often come out tasting astringent. For example, the high-extraction siphon method I posted a while ago only worked well with some specific roasts (some of which I listed in the post), and others came out very astringent regardless of whether channeling seemed to occur or not. Other examples include a few finely ground Aeropress and Buchner siphon brews I made that came out very astringent. I think some coffees may simply naturally contain a much lower amount of polyphenols, whether it is because of their varietal, terroir, processing or roasting. Grinding so fine ends up breaking most coffee cells, and therefore the chemical compounds are washed out by water (a process sometimes called erosion) rather than having to diffuse through the small pores in the cellulose walls of coffee cells. In that situation, polyphenols may very easily end up in the slurry, and if the coffee bed can’t filter them out, they may end up in the final brew even in the absence of channeling.
You may think that the same should happen with espresso and Turkish coffee, and you’re probably right. Assuming I’m not entirely mistaken about polyphenols extracting easily from broken coffee cells even in the absence of channels, I think either the high concentration or other things present in these types of brews (oils, suspended solids, etc.), may balance out the presence of polyphenols and make them less overwhelmingly astringent. That might also explain why it’s hard to take an evenly, highly extracted shot of espresso and dilute it into an amazing filter brew.
I think that one promising avenue may be to precipitate the polyphenols post-brewing by adding proteins in the finished coffee beverage, much like is routinely done in wine or beer making. Some things that are used for precipitation include egg whites, gum arabic, silica gels and a product called Polyclar (e.g., see this article about beer filtration) − these things are all rich in proteins. One potential major problem is that those compounds are typically left in the beer or wine for several hours to allow the precipitation to happen; if we wait this long with brewed coffee, it will probably taste very bad even if we remove all polyphenols from it.
I know this post probably opens up more questions than it answers, but I’m hoping it will help us think more clearly about what makes a brew taste astringent and how to avoid it !
I’d like to thank Scott Rao and Sylvain Mussigmann for useful comments.
Thanks for this scientific breakdown. Often hard to explain to people that sour is not the best description, this a far better way of explaining under extraction. I like the word and the description.
LikeLike
I might be missing sth, but I dont think this post relates astringency to under extraction.
If polyphenols are the cause of astringency and “polyphenols are … harder to extract from coffee particles, compared to smaller molecules like caffeine and the various acids we enjoy”, then astringency seems to be related more to over than under extraction.
LikeLiked by 1 person
Extraction is an average. Underextracted brews area at times locally overextracted because of channeling, making them astringent. Extraction is a hard term to use as an over/under since it can occur in different ways which might be hard to pinpoint.
LikeLiked by 1 person
That’s also exactly right
LikeLike
That’s right !
LikeLike
Thanks ! However astringency is usually a consequence of over-extraction. It can be localized over-extraction, in which case global EY can be lower – this often happens when bad channeling occurs.
LikeLiked by 1 person
Fascinating discussion. It would seem that fiber development will take much longer for filters to resolve polyphenols as you have examined – the culprit.
Enter Arabic Gum.
And perhaps one step beyond Arabic Gum, is Q-Naturale from the quillaja tree. About 12+ years ago, this was proving to be a more effective emulsifier. Quick search reflected some good academic research on comparing the two. It’s highly potent and some experimenting by pot or by cup would have to occur in a lab — and the questions would be: Would even ardent artisan coffee drinkers equip themselves to this degree, and what would be the return on the dollar / eye dropper / pin drop?
Some short reads:
https://www.ulprospector.com/en/na/Food/Detail/12852/368434/Q-NATURALE-200
https://www.beveragedaily.com/Article/2008/07/08/New-emulsifier-an-alternative-to-gum-arabic
LikeLike
Oh thanks ! My first experiment with arabic gum was a failure – and it made the coffee taste extremely sweet which isn’t great
LikeLike
The barista hustle blog post on channeling seems to suggest it’s the turbulence that causes increased extraction rate of tannin
https://baristahustle.com/blog/if-not-channelling-then-what/
LikeLike
Yeah, I’m very skeptical of that 🙂 turbulence ensures a much faster extraction, but it also ensures an extremely even extraction, so unless you over-extract everything, I don’t think it causes astringency. To the contrary, making extraction more even should allow a higher average extraction yield with no local over extraction anywhere. From experience turbulence can be a great thing.
LikeLike
I wouldn’t know cuz engineering isn’t my forté
the wiki does say the no slip condition thing doesn’t necessarily translate to real world and I guess in filter coffee, it’s not really a viscous liquid?
LikeLike
No-slip condition applies to coffee, but it doesn’t mean diffusion doesn’t happen. It only means there’s a thin layer of water around each particle that isn’t moving; chemicals can still diffuse through it.
LikeLike
Last reply seems to have disappeared but anyway
So the wiki page of the no slip condition seems to say it doesn’t 100% translate to real world anyway and filter coffee probably isn’t a viscous liquid, but interesting concept anyway
for all we know we will be brewing with enzyme-infused water to get the stuff we want and degrade those we don’t wanna
LikeLike
Yeah no-slip doesn’t always apply but in coffee brewing it should, maybe with the exception of very high agitation during pours, but even there it might still apply. Water is a viscous liquid, and filter coffee is a slightly more viscous liquid.
LikeLike
I’m rather confused by this talk of “channeling” for pour overs, especially when it’s stated as a fact that it occurs. Is there any proof that channeling even happens in a pour over? And if so how do you determine it? I’m talking about a normal brew within parameters, and not some wildly obvious disfunction.
LikeLiked by 1 person
It’s the best explanation we have for weak, asteingent brews. And intentionally causing a channel causes the same bad outcome. Actually seeing channeling is a hell of a challenge, I already asked a couple people that use functional MRIs if they could be used to see it, but the time resolution doesn’t seem good enough. It’s simply the best fitting model even if it’s not “proven”, and there’s just honestly no other model that can replicate the observations. On top of that, computer simulations based on the laws of physics predict that channels can and will happen.
LikeLiked by 1 person
When you say ‘channeling’ here do you mean what in an earlier post you called a “non-uniform flow”, and not a “real” channel physically dug by the water into the coffee bed (provoking an erosion)?
I mean, if the coffee bed is completely wet and you pour a lot of water over it, then presumably the water is flowing everywhere, just at different speeds in different places (but not only in some specific channels), right?
LikeLike
I mean both effects; if you displace some coffee particles you’ll make flow more uneven, and that would fit more under the “classical” channeling, but the boundary between the two is blurry
LikeLike
If it’s both weak (i.e. <17% EY) and astringent, I would say there was some flaw in methodology and recipe. I was more referring to typical astringent brews, for example using too fine grind / too high temperature (easier to do in a batch brewer) and so on. Isn't that just a case of overextraction rather than any kind of channeling or similar. You extracted more of the bad tasting chemicals than you intended to. You can also try tasting the last few drops out of a V60 for example and it will have this kind of burnt rubber and rather unpleasant taste.
LikeLike
Part or your comment disappeared but I restored it from my emails: “If it’s both weak (i.e. [less than] 17% EY) and astringent, I would say there was some flaw in methodology and recipe. I was more referring to typical astringent brews, for example using too fine grind / too high temperature (easier to do in a batch brewer) and so on. Isn’t that just a case of overextraction rather than any kind of channeling or similar. You extracted more of the bad tasting chemicals than you intended to. You can also try tasting the last few drops out of a V60 for example and it will have this kind of burnt rubber and rather unpleasant taste.”
I think simple over extraction is definitely a thing, and there’s definitely a grind size fine enough (for a fixed ratio) that will give you undesirable tastes and astringency. However, I don’t think it’s often the first cause of astringency in percolation brews (it probably almost always is the case in immersion brews because you can’t get channels). I used to think about brewing with the mental model you describe when there isn’t a clear big channel, but the experiment that completely changed my way to think about this is the cupping experiment by Barista Hustle where they showed that coffee particles don’t extract in their cores. This makes everything a lot more complicated than just global under- or overextraction, because the act of grinding finer doesn’t simply push the EY of all particles up in a uniform way, it also unlocks a lot of previously completely under extracted particle cores, which can now come in contact with water and extract. If you grind super fine like Ray Murakawa, then you are extracting every part of the coffee particles and in this particular scenario you can see the act of controlling the coffee/water ratio as a decision about which EY doesn’t taste over extracted for a given coffee bean (assuming the method is good enough to avoid channels). In the case of more typical V60s with coarser grind size, when you push things a bit finer you are not extracting each individual coffee cell faster, you are instead making more coffee cells available for extraction. This is why I think it’s hard to explain a small shift in grind size causing astringency without the appearance of channels in that situation.
LikeLike
I just came across your blog and I’m absolutely in awe! Two little thing: have you considered uCT instead of fMRI? As far as I remember it was good enough to check porosity of a bone. Or the interval between scans is too slow for this occasion?
Second thing I’m thinking about is bead filtering out polyphenols. Have you seem EM photographs of ground coffee? The cellulose is very highly porous it might be theoretically possible the there is some sweet spot where porosity is enough to filter out phenols and not enough to disrupt the walls.
LikeLike
Thanks ! I haven’t looked into uCT, I know very little about all of these 🙂 I wouldn’t be surprised if higher spatial resolution also means lower temporal resolution. I’ve looked into silica beads for filtration, but it seems that the relevant bead sizes are small enough to require laboratory safety equipment (goggles, mask etc) which isn’t practical for me 😅
LikeLike
I’ve just briefly checked the uCT idea https://www.researchgate.net/publication/309489812_In-situ_real_time_micro-CT_imaging_of_pore_scale_processes_the_next_frontier_for_laboratory_based_micro-CT_scanning it’s possible but in several years 🙂
Oh sorry, I misspelled bed as bed! I meant just that in coffee after grinding there are cellulose structures that may form sort of nanopores or tunnels that cause a resorption of polyphenols. Thet could hypothetically explain rebinding polyphenols in coffee bed (this bed that is nice when it’s flat in V60).
Yet the idea with some filter with beads or nanopores exactly the size matching polyphenols sounds both awesome and very expensive toy as for brewing standards, but we can dream 🙂
LikeLike
The Barista Hustle Kruve experiment seems somewhat flawed to me. We don’t know the proportions of the grind sizes identified vs the entire distribution. Even if the penetraton is as linear Perger summises, for a typical mid box extraction the particles up to +1stdev (maybe 1000um) in grind would be extracting to ~75%. Extraction level wasn’t particularly high in the experiment, certainly not high enough to suggest “everything” is being extracted.
Zanoni’s 92 experiment suggests that whilst the finer grinds (<250um) do extract further, larger grinds (up to 600um – not 600 Kruve, as Kruve & ISO/ASTM sieves don't produce the same results) still extract considerably more than Perger's linear model.
Can you expand on Ray Murakawa's technique please?
LikeLike
I agree, the real extraction probably happens in an exponentially decreasing way with layers rather than a on/off limit. The key however is not that observation, but rather that boulders would take a crazy long time to reach the same EY, indicating that some chemicals are effectively trapped inside the particle cores.
LikeLike
I just saw your comment was cut (because of the “less than” sign). Here’s the remainder of Mark’s comment: “whilst the finer grinds ( less than 250um) do extract further, larger grinds (up to 600um – not 600 Kruve, as Kruve & ISO/ASTM sieves don’t produce the same results) still extract considerably more than Perger’s linear model. Can you expand on Ray Murakawa’s technique please”
I’ll have a look at this paper’s results, thanks. It would be interesting to try and fit my exponential model to their data. Ray’s technique is basically this: grind turkish, do a V69 with a 18g dose (I think his ratio is 1:16), with 2 blooms. During each bloom you stir gently with a stick to remove all dry pockets, and then he pours the rest of the water in small stages, I think 20g each. He gets very high EYs but I haven’t tried it or tasted his brews. He often posts videos of this on the @melodripco Instagram profile.
LikeLike
Jonathan, thank you for this post!
How do you minimize astringency in your V60 brew (per your recipe, temp, and Rao/Perger water)? I think you have recommended doing the Rao spin only 1-2 times and pour slowly (~5g/s). Also, one can grind coarser, but sometimes this takes away from the flavor (notes) of the brew. On a side note: I’ve noticed I get more astringency with Ethiopian/African coffees (with my Baratza Forte) likely due to the more fines produced and longer draw down times. How do you combat this without sacrificing flavor? Thanks!
LikeLike
My pleasure ! Most of the time combating astringency is done by (1) not grinding too fine and (2) avoiding channels or clogging. Pouring from higher can help increase EY but if you pour too high you might clog your filter or dig a channel. Pouring faster has some of the same effects but it also plays on your total brew time. Pouring gently from a bit higher than usual works well for me but it might not for a grinder that is less uniform, so I recommend trying out for yourself. Ethiopians are definitely more tricky. I tend to pour from a bit lower, spin even more gently than usual, and sometimes I also grind coarser on top of that.
LikeLike
I use an AP Forte for grinding (No. 8, Alpha K) and a Melodrip over a V60 with a No. 4 Filtropa filter. I am a roaster. I don’t use a refractometer to call out a good cup. But because my beans – from Light to Espresso – are roasted between 18% and 25% development, my brew times for 8 oz water / 16g grind are close to 3:30.
They are without fines. It is a cup of nectar, whether light or dark, whether 16g or even deeper at 20g for light roasts.
I openly put this out because larger grind, low agitation, fresh roast, developed roast, will have a dominant effect on your cup. Does it pass the math of the refractometer or turbidity meter? I am not sure. Does it need to? If it didn’t taste very good, then yes. Does my air roaster have an impact? Maybe.
There are so many variables, but most of them can be learned and delivered for consistency.
LikeLike
Roasts definitely have a huge impact on brewing ! From what I hear, air roasters have a lot of potential.
LikeLike
„ I don’t use a refractometer to call out a good cup. “ It is sad so many people still think this way about refractometers. The only thing that can tell anyone what a good cup is are their senses, not a refractometer. A refractometer only reads TDS/strength of the beverage.
LikeLike
I agree that senses are most important and TDS alone will never tell you if your cup is good or not. It’s a useful navigation tool however, and can help you dial in. Much like a compass won’t tell you you made it out of the woods but it’s still useful to get there. Another thing I don’t get is why a simple tool makes some people so emotional 😅
LikeLike
There are a couple ways to reduce astringency; the main one is to grind coarser, but as you mention you can lose some brightness of flavors. Using a grinder with a more uniform particle distribution helped to grind finer without astringency in my experience. Depending on your grinder and the coffee, you might need to swirl very gently. For example, conical burrs or ethiopian coffee will produce a lot of fines and you’ll need to swirl much more gently to avoid filter clogging. If you see that your brew slows down a lot near the end, try swirling gentler, and if that’s not enough try also pouring from lower down with your kettle. If that’s still not enough, you might be forced to grind coarser, or go down the rabbit hole of using smaller doses (those are harder to do right in my experience, and require less agitation in general).
LikeLike
Interesting stuff. This model seems to line up with findings from the Hendon paper about grinding coarser. Does milk protein react with the polyphenols and cause precipitation, aka cappuccino?
LikeLike
What did you mean by “fresh water” vs “concentrated water” when you wrote the following? “Remember that fresh water is a much more potent solvent compared to concentrated water.”
LikeLike
I meant that the water near the bottom of the V60 is concentrated with coffee solubles, and therefore it is not as good at dissolving more stuff, compared to “fresh water” at the top of the V60 that is not yet concentrated with any coffee soluble.
LikeLike
It is not surprising that the cloudiness increased slowly at room temperature for 12 hours. Recristalization and precipitation of the dissolved compounds sometimes needs time to happen. Also if the container is open to the atmosphere, there should be evaporation of the water happening and this will contribute to the precipitation and recristalization. Highly doubt that it is related to protein binding with polyphenols.
LikeLike
The reason that makes me think it could have to do with polyphenols is that it happens even after a fast cool down to room temperature. Evaporation is really minimal because it’s happening in a small sealed container with almost no overhead. Do you have references I can read about cristallisation in the context of coffee ?
LikeLike
As I told the recrystallization and precipitation processes are sometimes slow. Cooling the solution fast doesn’t guarantee that both processes will happen immediately. The coffee drink is a complex solution and a lot of processes and variables can trigger. After 30 min the concentration of precipitates in the solution decreases so the process of precipitation slows down. This process couldn’t just finish because this is an equilibrium. The equilibrium will be changed but both processes (precipitation and dissolution) will still be happening.
The same applies also for recrystallization.
I have to look for a reference for crystallization (sorry for the misspelling in my first comment) in coffee. But 100% sure it will happen. For example, the caffeine is very soluble in water so decreasing the temperature and evaporating the water will lead to caffeine crystallization. This applies also for every other dissolved compound in the coffee. Depending on the solubility and chemistry all components will crystallize or precipitate at one point.
The increasing cloudiness could be because of many reasons. It could be the formation of colloids, oxidation processes, chemical decomposition, particle aggregation, etc.
LikeLike
Interesting, thanks! Evaporation isn’t the culprit in this case (sealed) but it’s possible that other changes like precipitation occur slowly even after temperature is stable.
LikeLike